12+ Chapter 8 Covalent Bonding Answer Key
Web Write a balanced equation describing each of the following chemical reactions. The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond.
Chemistry Chapter 8 Covalent Bonding Flashcards Quizlet
Web We would like to show you a description here but the site wont allow us.

. Web The Octet Rule. 186 Occurrence Preparation and Properties of Carbonates. However as noted previously in this chapter such conditions are not realistic.
One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Web where w max w max refers to all types of work except expansion pressure-volume work. Which of the following compound contains both covalent and ionic bond.
ASEXUAL CELLS EVOLUTION STIMULI DEVELOPMENT. Web A plot of A versus t for a zero-order reaction is a straight line with a slope of k and a y-intercept of A 0Figure 1211 shows a plot of NH 3 versus t for the thermal decomposition of ammonia at the surface of two different heated solids. 185 Occurrence Preparation and Compounds of Hydrogen.
Web Dissertation Paper Writing Service. Web Coordination Compounds Class 12 Notes Chapter 9 Coordination compounds is a challenging area in modern inorganic chemistry. Modification of work by the Italian voiceFlickr.
A Solid potassium chlorate KClO 3 decomposes to form solid potassium chloride and diatomic oxygen gas. 183 Structure and General Properties of the Metalloids. Web Gradescope allows me to give a short quiz every day in my section of 60 students and grade them all on my 30 minute train ride home.
Covalent bonding will be there in between carbon and chlorine. The other halogen molecules F 2 Br 2 I 2 and At 2 form bonds like those in the chlorine molecule. Web The remaining elements of this group show a formal oxidation state of 3 in their covalent compounds.
A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms attain their nearest inert gas configuration. C When solid sodium chloride is added to aqueous sulfuric acid hydrogen chloride gas. Web Answer Key.
Covalent Bonding and Electron Shells. By referring to the NCERT Solutions for Class 12 Chemistry Chapter 7 you can answer both intext and. Web a displaced water volume 28 mL.
Web The attractive force which holds together the atoms or group of atoms in a chemical species is known as a chemical bond. B displaced water mass 28 g. The extent of hydrogen bonding in P H 3 PH_3 P H 3.
Enthalpies of combustion for many substances have been measured. Web Water is the chemical substance with chemical formula H 2 O. In addition the technologies used to extract work from a spontaneous process eg batteries are never 100 efficient and so the work done by these processes is always less than the.
Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. The decomposition reaction exhibits first-order behavior at a quartz SiO 2 surface as suggested by the exponentially. A gradual but visually impressive change spontaneously occurs as the initially colorless solution becomes increasingly blue and the initially smooth copper wire becomes covered with a porous.
Definitions Relationship the Octet Rule. The tendency of main group atoms to form enough bonds to obtain eight valence electrons is. C A covalent bond present between N and C Atom and ionic bond present between Na ion and NC ion.
Web What is Dynamic Equilibrium. Very Short Answer Type Questions Page no. One single bond between atoms and three lone pairs of electrons per atomThis allows each halogen atom to have a noble gas electron configuration.
Web Free PDF download of NCERT Solutions for Class 11 Chemistry Chapter 12 - Organic Chemistry - Some Basic Principles and Techniques solved by Expert Teachers as per NCERT CBSE textbook guidelines. Web That being said here we are providing a set of NCERT Solutions for Class 11 Chemistry Chapter 9 to help students test their knowledge about the chapter which is hydrogen and they can also learn how to work on the questions as well. Dynamic Equilibrium can be defined as the state of a given system in which the reversible reaction taking place in it stops changing the ratio of reactants and products but there is a movement of.
3205 10 7 m Bohrs model of the hydrogen atom provides insight into the behavior of matter at the microscopic level but it does not account for electronelectron interactions in atoms with more than one electron. 182 Occurrence and Preparation of the Representative Metals. The definition of the chemical bond as a shared electron pair could be extended to describe the dative bond and the elaboration of Lewis acidbase interactions.
The atomic numbers of four elements ABC and D are 6810 and 12 respectively. We Offer the Custom Writing Service with 3 Key Benefits. FTCE Physics 6-12 032 Prep.
With the help of our downloadable Solutions for NCERT Class 11 Chemistry Chapter 9 PDF students can access the. Web Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. 6198 10 19 J.
Human Resource Development Plan. The two elements which can react to form ionic bonds or ionic compounds. Web Access Lakhmir Singh Solutions for Class 10 Chemistry Chapter 3.
If the atoms that form a covalent bond are identical as in H 2 Cl 2 and other diatomic molecules then the electrons in the bond must be shared equallyWe refer to this as a pure covalent bondElectrons shared in pure covalent bonds have an equal probability of being near each nucleus. Web As demonstration of spontaneous chemical change Figure 172 shows the result of immersing a coiled wire of copper into an aqueous solution of silver nitrate. A few of these are listed in Many readily available substances with large enthalpies of combustion are used as fuels including hydrogen carbon as coal or charcoal and hydrocarbons compounds containing only hydrogen and carbon such.
A chemical bonding between the two atoms which shares a single pair of an electron is. C The block mass is 276 g essentially equal to the mass of displaced water 28 g and consistent with Archimedes principle of buoyancy. Web Standard enthalpy of combustion.
The students getimmediate custom feedback that helps them understand how theyre doing in the classimmediate custom feedback that helps them understand how theyre doing in the classand helps me monitor. Modification of work by vxlaFlickr. B Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6.
184 Structure and General Properties of the Nonmetals. In addition to the 3 state N and P also show 1 and 2 oxidation states. Web What is an Ionic Bond.
All Chapter 12 - Organic Chemistry - Some Basic Principles and Techniques Exercises Questions with Solutions to help you to revise. Due to the advancement in this area new concepts such as models of bonding molecular structure important insights on the functioning of complex components of the biological system etc.

Chemistry The Science In Context Volume I And Ii 4th Edition Gilbert

Chemistry The Science In Context Volume I And Ii 4th Edition Gilbert

Valence Bond Theory Need Postulates Limitations Examples And Videos

Chapter 8 Study

Chapter 8 Redox Reactions Ppt For Class 11 Cbse

Pdf Dynamic And Static Nature Of Br 4 S 4c 6e And Se 2 Br 5 S 7c 10e In The Selenanthrene System And Related Species Elucidated By Qtaim Dual Functional Analysis With Qc Calculations

Chemistry Revision Notes 2012 Pdf

How To Use This Presentation Ppt Download
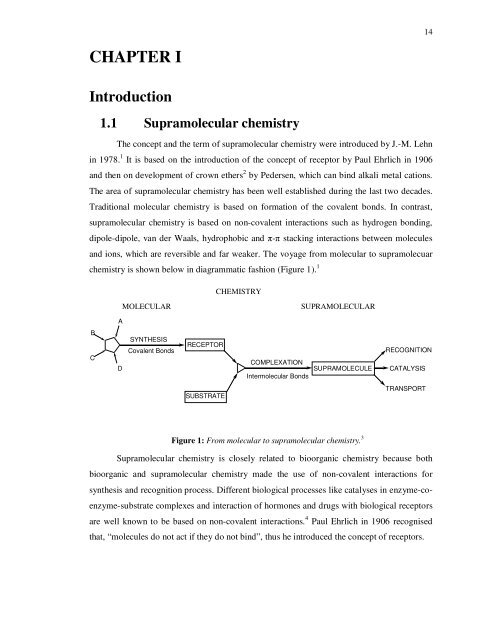
Introduction

Chapter 7 8 And 9 Study Guide Ionic Bonding ÿ Covalent Bonding

How To Use This Presentation Ppt Download

Worksheet Drawing Ionic Covalent Bond Diagrams 2 Worksheet Set

Chapter 8 Covalent Bonding Ppt Video Online Download
Why Is Bcl3 An Electron Deficient Compound And Why Does It Have A Strong Tendency To Gain An Additional Pair Of Electrons By Reacting With Species With A Lone Pair Of Electrons

Extra Practice Covalent Bonding W Key

Chapter 8 Covalent Bonding Flashcards Quizlet
Pdf High Pressure And Chemical Bonding In Materials Chemistry